Research
Research in our lab focuses on exploiting cutting-edge chemical, biomaterial and nanomedicine technologies to understand physiological responses during disease and regeneration and fulfill critical unmet needs in the clinic. We use chemical prodrug therapy, controlled drug delivery, biomaterials, and nanomedicine to enable new forms of cancer chemotherapy and immunotherapy as well as treatment of infection and other diseases.
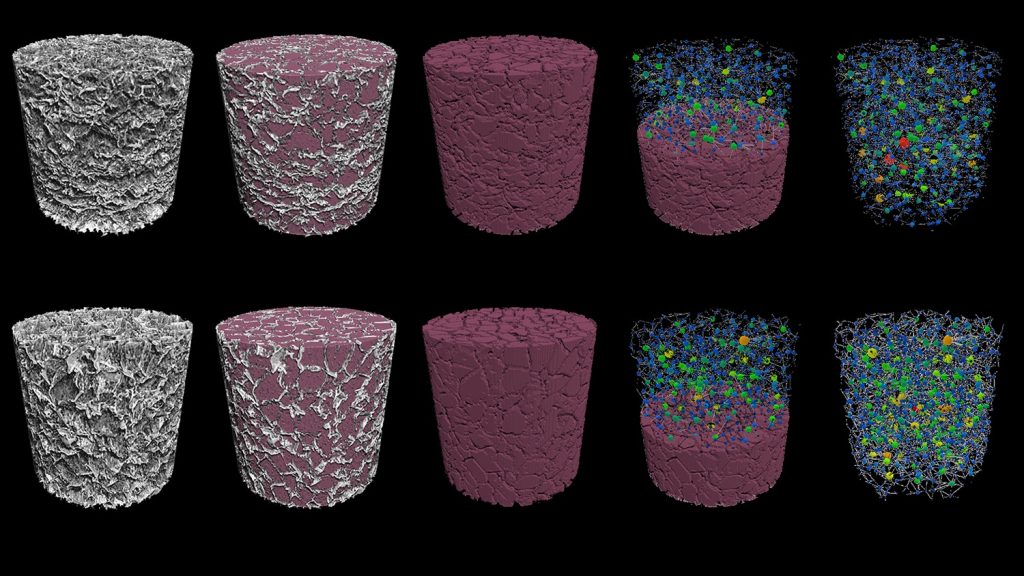
Novel biomaterials for viral transduction. Chimeric Antigen Receptor (CAR) T cells are T cells genetically engineered to recognize and destroy cancerous cells. CAR T cell therapy has revolutionized the treatment of some hematological malignancies, and it has the potential to revolutionize the treatment of solid cancers. However, due to the lengthy (>28 days) manufacturing process, a single dose of CAR T cells costs hundreds of thousands of dollars. In hopes of dramatically lowering this treatment’s cost, our lab researches biomaterials that could shorten the production of CAR T cells from weeks to mere hours. These algae-based, dime-sized scaffolds capable of manufacturing CAR T cells inside the body could streamline the production of a variety of genetically engineered cells used in immunotherapies. Our ultimate vision is to implant these scaffolds during an out-patient procedure that catalyzes the body to produce its own CAR T cells naturally.
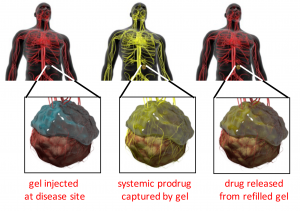
Replenishable drug delivery systems. One major direction in the lab centers on developing replenishable drug depots, which allow noninvasive refilling of drug-eluting depots in vivo. We take advantage of nucleic acid binding, bioorthogonal click chemistry, and bio-recognition to capture drug payloads from the blood, including small molecule drugs and nanoparticles. We have demonstrated that replenishable depots can capture therapeutics from circulation. The depot devices retain their ability to capture therapeutics from circulation for months and can be refilled numerous times. More recently, we have created non-toxic, “prodrug” therapeutics that are hydrolytically converted into active molecules inside our refillable devices.
The Brudno lab is expanding the replenishable depot technology for use with new chemotherapies such as carboplatin and paclitaxel for targeting locally recurrent peritoneal cancers such as liposarcoma and ovarian cancer. We are also exploring combining replenishable intratumoral depots with blood-brain barrier disruption for targeting gliomas, including glioblastoma. The lab is also actively researching combining replenishable depots with immunotherapy to improve safety and efficacy of this promising technology.
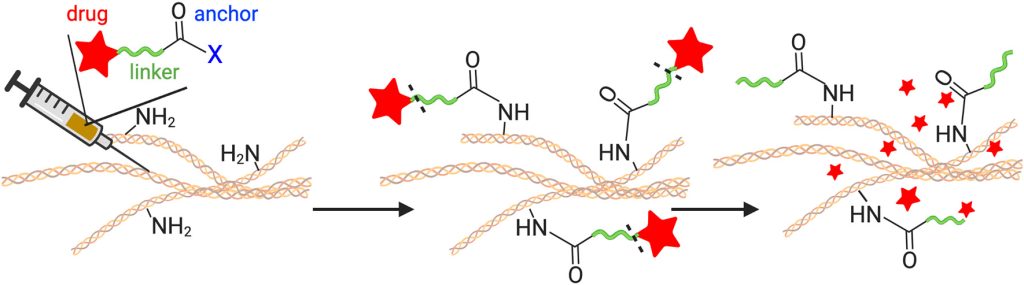
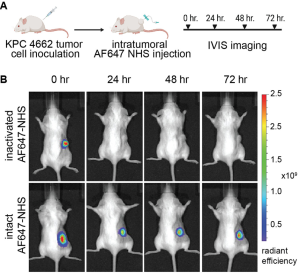
Materials-free drug delivery systems. Sustained, local delivery of therapeutics could revolutionize the care of patients by reducing toxicity and rapid clearance. Unfortunately, existing local delivery technologies require extraneous materials such as scaffolds or implants to be placed at the target site. These materials limit the use of these local drug depots, making them impractical in many parts of the body, including the brain, stiff tumors, fibrous tissues, and joints. Recently our research group developed a novel, materials-free sustained release technology, termed Tissue-Reactive Anchoring Pharmaceuticals (TRAPs). The TRAPs technology takes advantage of tissue structures by attaching drugs through a covalent bond directly at the site of administration. Importantly, the drug is attached via a robust and tunable chemical linker that slowly dissolves, for sustained release into the tissue. Overall, this invention provides a flexible drug delivery platform amenable to many drug classes for the treatment of cancer, infection, inflammation, and many other diseases.